News
Video Highlight of Research from UVA VPR
Fri, 04/12/2024
As a part of the Research Communications Fellow program, Charles has recorded a video highlighting the current work in the group!
Collaboration with Hilinski Lab Published in JACS!
Fri, 03/15/2024
Congratulations to Emma, who collaborated with Anna Davis of Mike Hilinski's group, on publishing her study on electrifying an organocatalyst for dioxygen reduction in JACS!

Emma and Ian's paper published in Chemical Science!
Tue, 02/13/2024
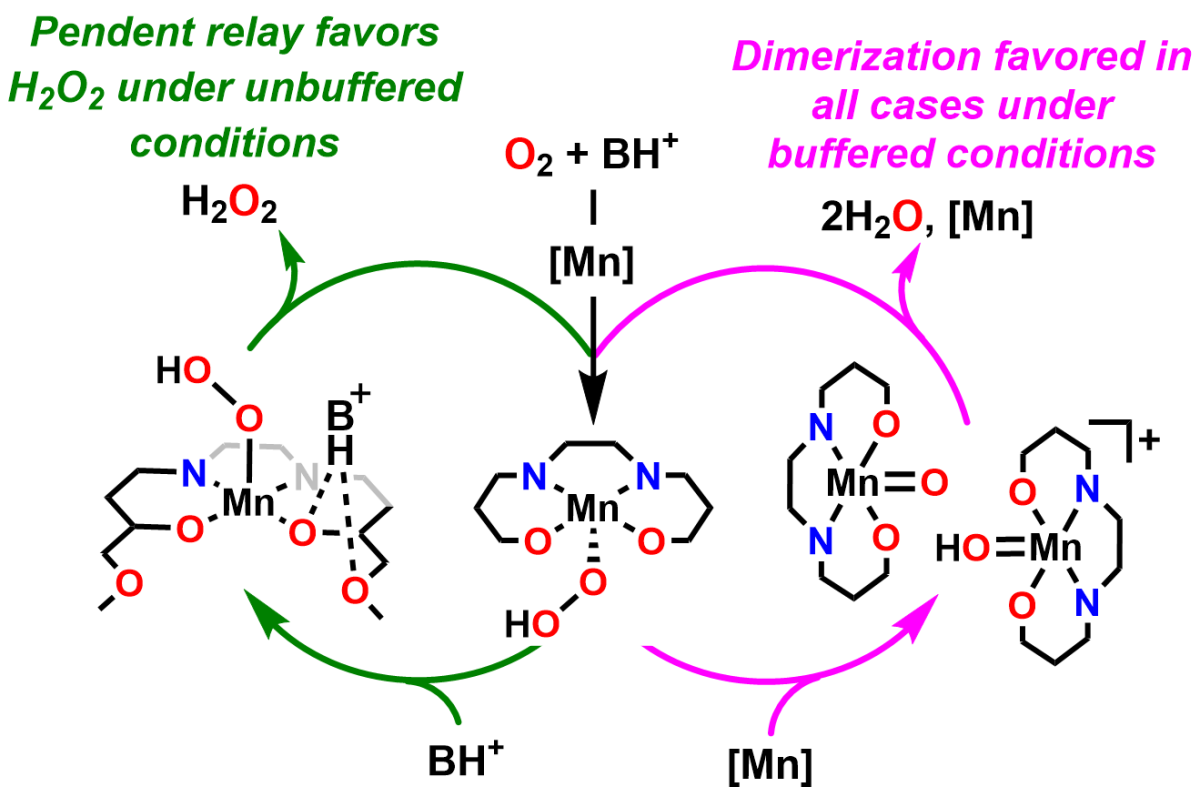
Graduate researcher Emma and former undergraduate researcher Ian had their paper on Mn-based ORR catalysts with pendent relays published in Chemical Science as an Edge Article recently, congratulations!
Collaborative Papers Published in J. Chem. Phys. and ACS Applied Material Interfaces!
Mon, 11/20/2023
Collaborative papers with the Giri Group in UVA Chemical Engineering and Prof. Dan Harrison at VMI have appeared online! Congratulations to all the authors!
Verma, P.K.; Koellner, C.A.; Hall, H.; Phister, M.R.; Stone, K.H.; Nichols, A.W.; Dhakal, A.; Ashcraft, E.; Machan, C.W.; Giri, G. “Solution Shearing of Zirconium (Zr)-Based Metal–Organic Frameworks NU-901 and MOF-525 Thin Films for Electrocatalytic Reduction Applications”ACS Appl. Mater. Interfaces 2023, DOI: 10.1021/acsami.3c12011.
Reid, A.G.; Moberg, M.E.; Koellner, C.A.; Machan, C.W.#; Thornton, D.A.; Dickenson, J.C.; Stober, J.J.; Turner, D.A.; Tarring, T.J.; Brown, C.A.; Harrison, D.P.# “Sterically Attenuated Electronic Communication in Cobalt Complexes of Meridional Isoquinoline-Derived Ligands for Applications in Electrocatalysis” J. Chem. Phys. 2023, DOI: 10.1063/5.0174177.
Connor's Paper Published in Chem Commun!
Tue, 04/25/2023
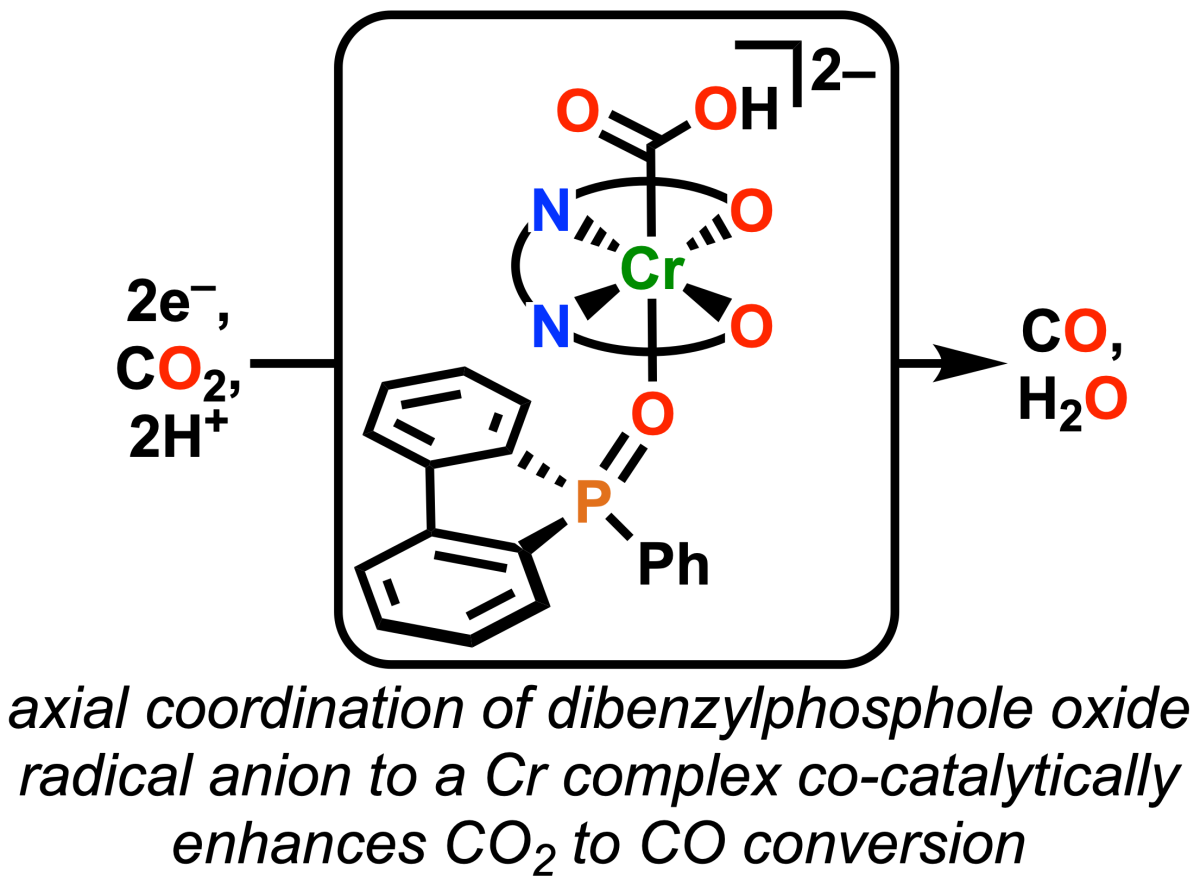
Congratulations to Connor, who (with help from Amelia) has published a paper in Chem. Commun. on the use of a dibenzophosphole oxide as a redox mediator! Our interest in the dibenzothiophene dioxide redox mediator platform led us to explore a related phosphorus-based system. The phosphine-based mediator coordinates the Cr center relatively more strongly than the sulfur-based one, but loses some specifcity in its binding orientation.